This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
🌟 Improve your sports field with a expert audit.










2 Responses
How would I measure potassium in the soil using the Laquq Twingo K+ and if so how would I acquire it in Peru?
Dear José Antonio,
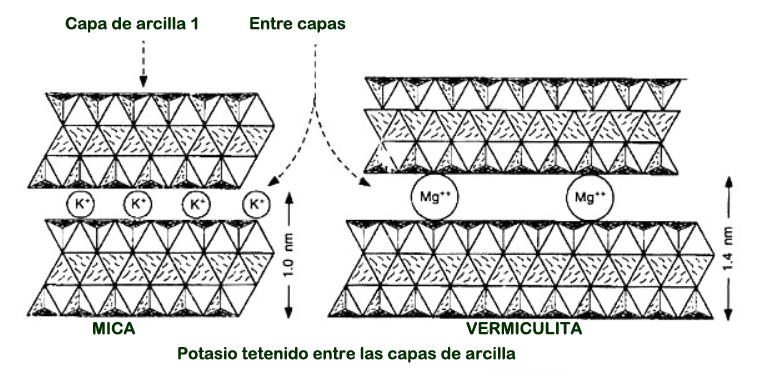
Potassium in soil can be measured by making a solution of soil in distilled water. The sensor is introduced into the liquid and the value is obtained.
This measurement will make it easier for you to manage potassium in soil. There are instruments that can tell you the concentration of many ions such as nitrogen, phosphorous, potassium, calcium, etc.
Best regards.